Definition: Redox Reaction
This article will cover Redox reaction, reduction, oxidation and should help you get a good mastery of what a redox reaction is all about.
Redox in most cases has always been associated as the addition of hydrogen to a compound. Whereas, oxidation as the addition of oxygen to a compound. In effect, be a redox reaction is more of the adding or the loss of electrons (actual or formal transfer of electrons)
Oxidation and reduction
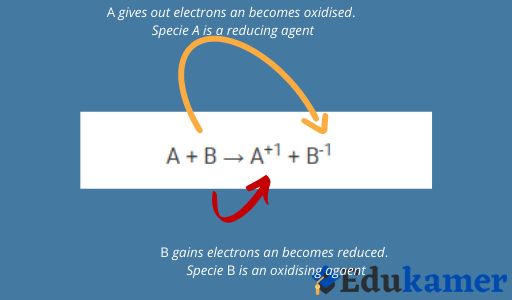
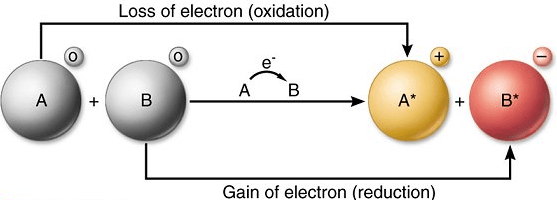
During an redox reaction, both oxidation and reduct takes places between species and the species which gains electrons is called the oxidant and is been reduced at the end of the reaction.
Conversely, the species hich gives out electrons and becomes Oxidised is called the Reductant.
This therefore implies that oxidation and reduction could be described as the removal of an electron and the addition of an electron respectively from a substance.
In actual reaction processes, electrons are not added or removed. Instead, combinding power or oxidation states changes thus reduction is a decrease in oxidation number whereas oxidation is an increase in oxidation number.
So,in a redox reaction, we have two species;
- The reducing agent
- Oxidising agent
Oxidising agent
An oxidising agent or oxidant is a compound or chemical species which gains electrons during a chemical reaction. Its oxidation number reduces during the process.
A good example of an oxidising agent include halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H2O2)
Reducing Agent
Reducing agent or reductant is an element or compound that loses an electron to an electron recipient in a redox chemical reaction. A reducing agent is thus oxidized when it loses electrons in the redox reaction. Reducing agents “reduce” oxidizing agents.
Common reducing agents include metals potassium, calcium, barium, sodium and magnesium, and also compounds that contain the H− ion, those being NaH, LiH, LiAlH4 and CaH2.
A Redox reaction
Definition: A redox reaction is a reaction in which oxidation and reduction are occurring simultaneously. In other words, it is a reaction one species undergoes reduction whereas the other undergoes oxidation.
Types of redox reactions
There are different types of redox reactions. Depending on the kind of reactions involve we have;
- Decomposition Reaction
- Combination Reaction
- Displacement Reaction
- Disproportionation Reactions
A decomposition Reaction is a type of redox reaction where a coupond is broken down into compounds.
A good example is the decomposition of Sodium Carbonate at at 400° C.
Na2CO3 → Na2O + CO2
A combination reaction is the reverse of a decomposition reaction. In this type of redox reaction. Here, two or more compounds to form a single coumpound.
4Fe (S) + 3O2 (g) → 2Fe2O3 (s)
For a disproportionation reaction, a single species undergoes both reduction and oxidation. For example, the reaction which occurs when Chlorine is dissolved in water.
Cl2 (g) + H2O (l) → HCl (l) + HOCl (g)
In the raction above, Chlorine undergoes both oxidation and reduction. The Chlorine in the HCl undergoes and reduced of oxidation state. On the other hand, the Chlorine in the molecule of Hypochlorous acide has and oxidation number of +1 and is oxidised.
When an atom or an ion in a compound is replaced by an atom or an ion of another element the chemical reaction is called a displacement reaction.

